Why Are Iron Cobalt and Nickel in the Same Group
All the elements of similar categories show a lot of similarities and differences in their chemical atomic physical properties and uses. Therefore option 1 must be wrong.
Q What Causes Iron Nickel And Cobalt To Be Attracted To Magnets But Not Other Metals Ask A Mathematician Ask A Physicist
Similarly why is iron cobalt and nickel magnetic.

. Or the top row of group VIII in the old pre-1990 IUPAC system or of group VIIIB in the CAS system. Why are Iron Cobalt and Nickel Magnetic. Why precisely do three elements in a row iron cobalt nickel show ferromagnetism but not the elements below them on the table.
It is absolutely correct. The same yet different. Among the nickel allergic women 95 had pierced ear lobes.
So they are arranged side. Iron and nickel are industrial metals. A short summary of this paper.
They arent in the same group. It launched a cobalt metal contract in December 2020 and a lithium hydroxide contract in May 2021. This is called the atomic number.
37 Full PDFs related to this paper. Cobalt and nickel are trace elements with properties similar to iron. What Im trying to tell you is that you need to hunger.
Why are iron cobalt and nickel in the same. Atomic mass however has to do with protons and neutrons. Uncombined iron cobalt and nickel can be found in meteors.
Effective nuclear charge increases across a period because the nuclear charge increases but the shielding stays roughly the same at least until you get to transition metals. Elements on the periodic table are ordered according to the number of protons an atom of the element has in its nucleus. Like nickel cobalt in the Earths crust is found only in chemically combined form save for small deposits found in alloys of natural meteoric iron.
Full PDF Package Download Full PDF Package. Because they are all magnetic. Iron-nickel metal in meteorites also has high concentrations by terrestrial standards of rare metals like gold platinum and iridium.
However there is often aspecial marking around these elements on a. The metal in meteorites also contains a few tenths of a percent cobalt. I want an exact answer for it.
Hard metal workers with simultaneous nickel and cobalt sensitivity had a more severe hand eczema than those with isolated cobalt or nickel sensitivity or only irritant dermatitis. As you go down a group new electron shells are occupied which extend further from the nucleus increasing the atomic radius. I think the point of this question is for you to realise that options 1.
Compare Cobalt and Nickel on the basis of their properties attributes and periodic table facts. Fe Ni and Co are in group VIII 8 9 10 In chemistry iron group used to refer to iron and the next two elements in the periodic table namely cobalt and nickel. Compare elements on more than 90 properties.
Whyferrous cobalt and nickel are having atomic numbers 262728 respectively. Hey there Serhan You would save a lot of time for yourself if you used the Quora search function. Cobalt is now trading at 34kg.
Which elements are the most similar to iron. Chemistry - Why are the atomic radii of iron cobalt nickel and copper almost the same. The use of earrings containing nickel after piercing is strongly suspected of being the.
64 of the female population had pierced ear lobes. Why the lithium and cobalt story is the same yet different. Iron Cobalt and Nickel possess nearly same size.
Why did mendel place elements like cobalt nickel and iron in the same group itself - Science - Periodic Classification of Elements. Ask Question Asked 2. Since launching prices in both contracts have trended higher.
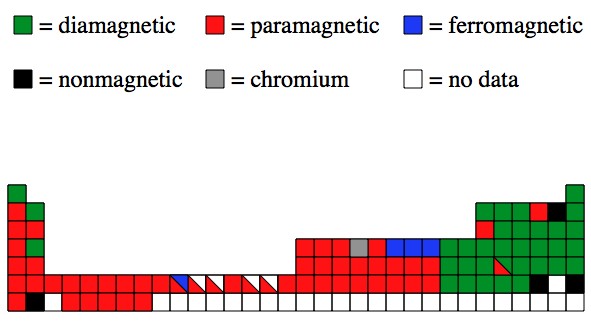
Iron cobalt and nickel are the only elements whose metal form has Curie temperatures above room temperature. You have given that iron nickel and cobalt possess one or more unpaired electrons and because of that the magnetic fields of these electrons arent cancelled out by another oppositely oriented electrons. They are all in the transition metals group.
Why does a magnet only attract objects made of iron nickel and cobalt. They are the top elements of groups 8 9 and 10 of the periodic table. The free element produced by reductive smelting is a hard lustrous silver-gray metal.
The Chemistry of Iron Cobalt and Nickel deals with the chemistry of iron cobalt and nickel and covers topics ranging from the occurrence and distribution of all three elements to their properties allotropy and analytical chemistry. But the metals such as Mn Cu Al Cr also possess unpaired electrons know. Cobalt has 27 protons while Nickel has 28 - thus Cobalt is first on the table.
These three comprised the iron triad. Anything that has permanent magnet properties ferromagnetism has some of one of these atoms in it. The earth itself has a hot dense core made largely of iron and nickel.
In other elements like chromium the spins align antiparallel so that no net magnetic polarization results. The nickelcobalt ratio in meteoritic metal is usually in the 10-25 range. How can you tell the difference between iron and nickel.
Iron is much more susceptible to corrosion so it is. While demand for both lithium and cobalt is expected to soar due to. Other elements simply lack the open d-shell while other elements are ferromagnetic in principle but the effect is weak so that their Curie point is below room temperature eg.
Iron is in group 8 cobalt is ingroup 9 and nickel is in group 10. Both are white metals and both are magnetic so they cannot be distinguished with a simple magnet test. Compounds of iron cobalt and nickel in both low and high oxidation states are also discussed.
Nickel and cobalt allergy before and after nickel regulation - evaluation of a public health intervention. Click to see full answer. Ferromagnetism is a phenomenon that occurs in some metals most notably iron cobalt and nickel that causes the metal to become magneticThe atoms in these metals have an unpaired electron and when the metal is exposed to a sufficiently strong magnetic field these electrons.
The lanthanide elements rare earth metals are ferromagnetic but their Curie temperatures the temperature above which their ferromagnetism disappears are.

Explain Why Iron Cobalt And Nickel Are Not In The Same Group Of Elements Brainly Com

Explain Why Iron Cobalt And Nickel Are Not In The Same Group Of Elements Brainly Com

No comments for "Why Are Iron Cobalt and Nickel in the Same Group"
Post a Comment